Congratulations to Maodi Zhang and Teng Wang for their paper accepted by Journal of the American Chemical Society!
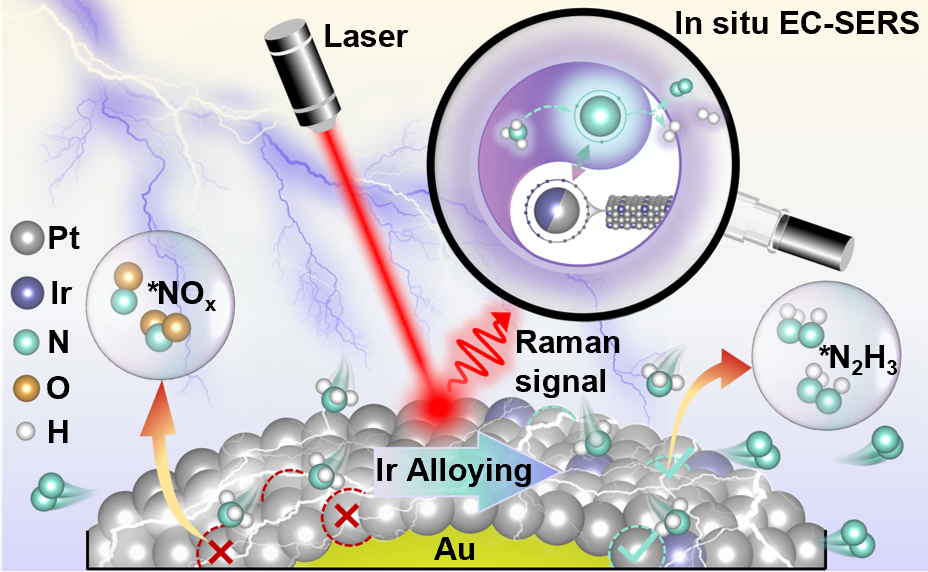
The electrochemical ammonia oxidation reaction (AOR) is a cornerstone technology for sustainable on-site hydrogen production and clean energy conversion. While Pt-based alloys such as PtIr have emerged as promising AOR electrocatalysts to address the rapid deactivation of pure Pt systems, the atomic-level mechanisms governing their superior stability remain unresolved. Here, we employ in situ electrochemical surface-enhanced Raman spectroscopy to directly track the evolution of intermediates on Pt and PtIr catalysts during the AOR. On pure Pt electrodes, increasing potential drives sequential oxidation of *NH2 to *N3–, ultimately forming surface-bound *NOx species that poison active sites and degrade performance. In stark contrast, PtIr alloys bypass this detrimental pathway by enabling an unconventional *N2H3-centered mechanism. Through N–N coupling of *NH and *NH2 intermediates at Pt–Ir integrative catalytic sites, *N2H3 is generated and dehydrogenated to N2 without the formation of toxic byproducts. Therefore, PtIr alloy catalysts exhibit significantly enhanced durability compared to monometallic Pt catalysts. This work highlights the paramount importance of directly observing key reaction intermediates via in situ spectroscopy for elucidating catalytic mechanisms.