-
When light interacts with a noble metal nanoparticle, the free electron gas in the metal is externally driven to perform oscillations at its plasma eigenfrequency (ω0). This resonant excitation of electrons (localized surface plasmon resonance) generates in close vicinity of the metal surface a high-frequency electromagnetic vibration, i.e. an enhanced optical field on the nanoparticle.
Let us assume the increased local electric field amplitude on the metal surface is Eloc(ω0), where ω0 is the plasmon resonance frequency. If a molecule is located inside the local field, the generated Raman scattering can be elastically scattered off the metal surface. In other words, both the incident light (E0) and the Raman scattering are enhanced via elastic scattering off the metal nanoparticle. The intensity of Raman scattering from the molecule is proportional to the square of the amplitude at ω0. And this scattering radiation is again amplified to an intensity that is proportional to the square of the amplitude at ω0 ± ωR. Overall, the intensity of the enhanced Raman scattering (ISERS) is enhanced two times by the metal substrate:ISERS ~ |Eloc(ω0)|2 x |Eloc(ω0 ± ωR)|2For ω0 » ωR, the SERS intensity can be approximated by the fourth power of the local electric field amplitude on the metal surface:ISERS ~ |Eloc(ω0)|4This equation is called the |E|4 approximation for the SERS enhancement (which is a good approximation only for the near infrared excitation): a moderate enhancement of E results in a huge SERS signal enhancement (|Eloc|4).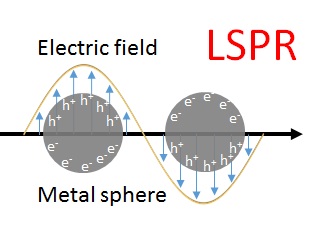
Background 【+】
Area of Interest
-
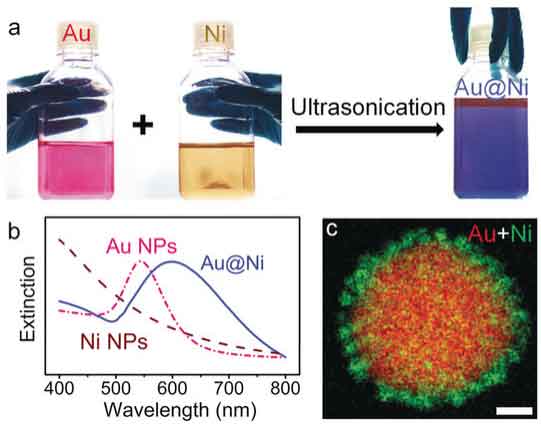
Rational design of bifunctional nanoparticles
SERS monitoring of nanoparticle-catalyzed reactions requires bifunctional substrates with both SERS and catalytic activities. Unfortunately, conventional catalysts are not SERS-active. For example, small Au nanoparticles that are catalytically active are not SERS active due to the very small scattering cross-sections. In our group, we design and synthesize nanoparticle hybrids and assemblies (superstructures) using plasmonic metals together with catalytically active materials. The chemical reactions on the catalyst surface can be monitored under real reaction conditions by SERS.
-
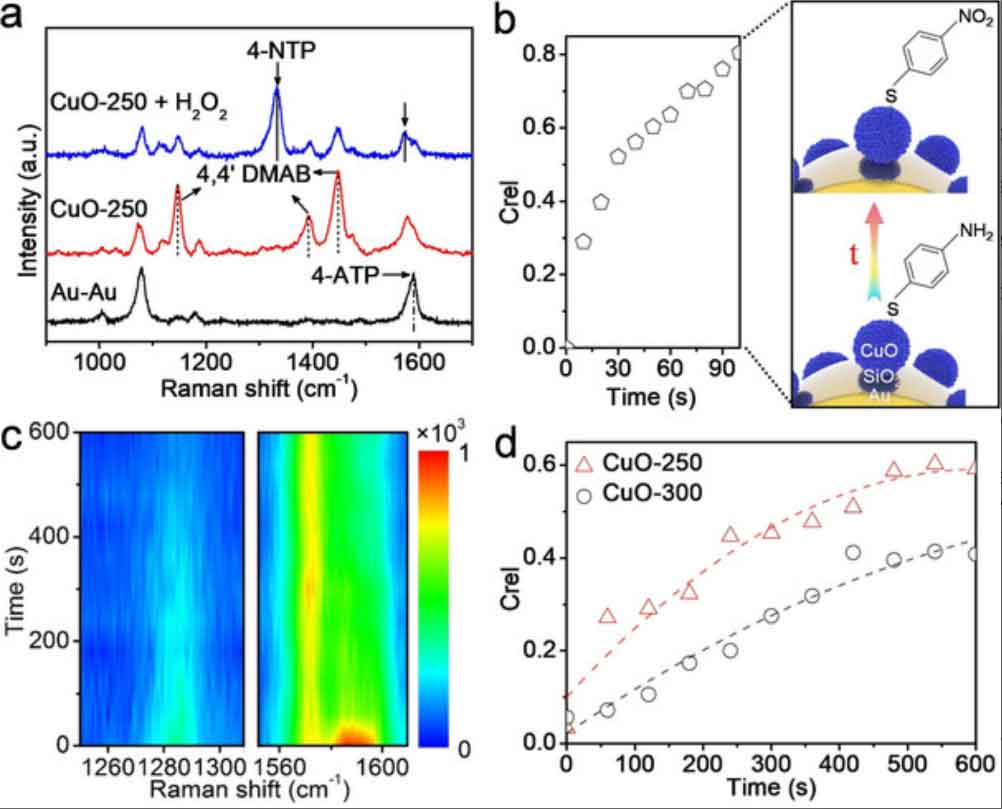
Label-free monitoring of catalysis
Via in situ SERS, the different components at the catalytic interface can be detected simultaneously. We investigate not only the molecular structural changes during the reaction but also the reaction kinetics under different reaction conditions. To ensure that the reaction occurs under the required conditions, i.e. temperature, pressure, pH, and solvents, the in situ sample cells/reactors have to be designed according to the reactions. We aim at establishing a non-destructive in situ characterization platform for mechanistic study of nanoparticle-catalyzed reactions.
-
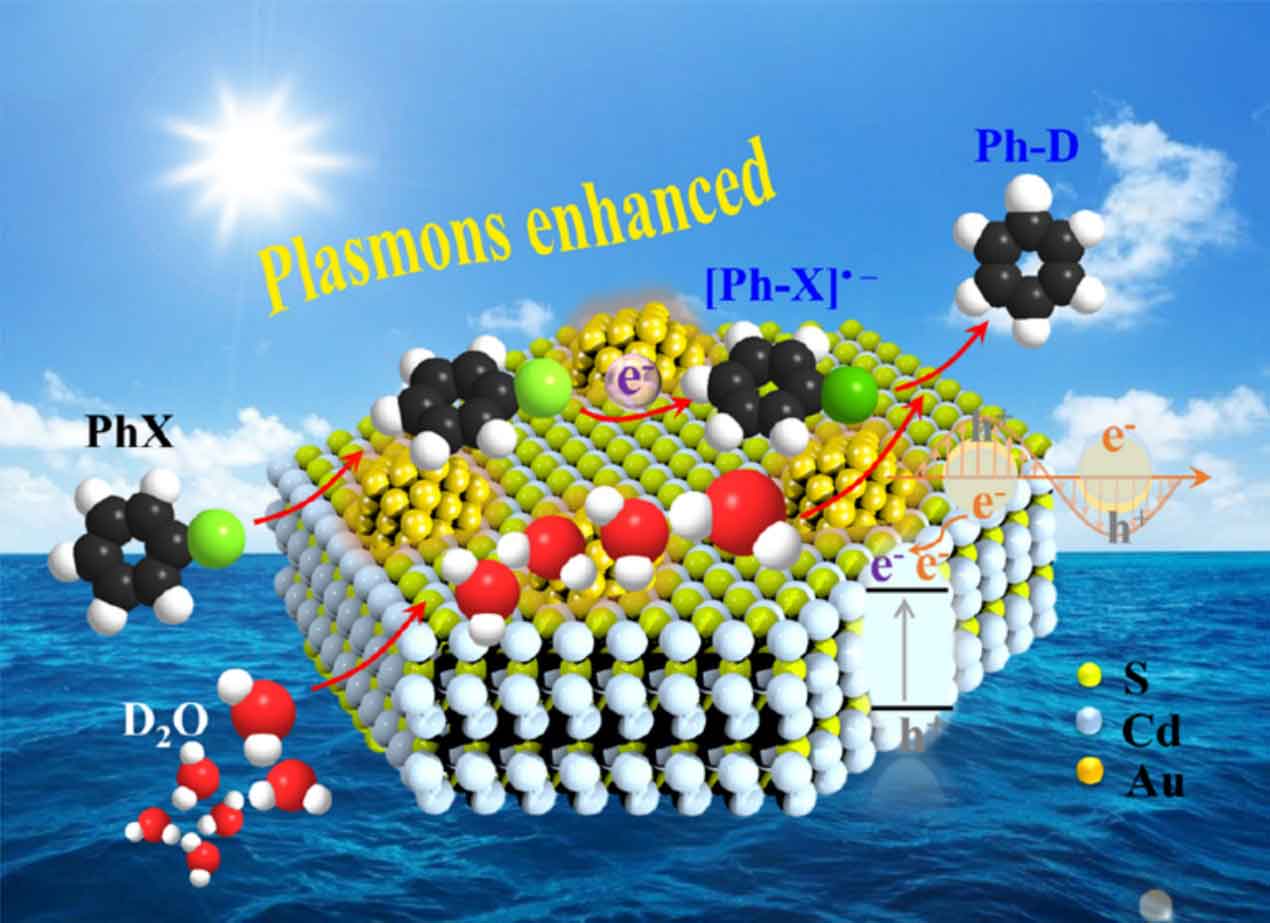
Plasmon-induced chemistry
Hybrid plasmonic nanostructures are synthesized for plasmon-induced chemical transformations. When plasmons are excited on the metal surface, there are two fundamentally different decay channels: the radiative channel, in which the metal nanoparticle acts as a nanoantenna (scattering), and the non-radiative channel (absorption). The absorption of photons may lead to the generation of heat by electron–phonon coupling or to the generation of charge carriers by the excitation of electron–hole pairs. In the latter case, the generated high-energy electrons can be transferred from the surface of the metal to an adjacent electron acceptor, e.g. a semiconductor or a molecule.